Soil and Water pH - Part 2
Horticultural methods, techniques, growing practices, and principles
The pH of soil and water and how it affects plant growth and chemical makeup of soils and media is somewhat detailed. Over the course of five newsletters, we will present different aspects about pH. Once complete, one should have a thorough understanding of pH and how it influences plant production.
Part I – What is pH?
Part II – How is nutrient availability affected by pH?
Part III – How is pH affected by fertilizers?
Part IV – How to adjust pH of soils
Part V – How to adjust pH of irrigation waters
As I mentioned in the fall issue, the pH of soil and water is one of the most important chemical factors to consider aside from nutrient concentrations since soil pH influences nutrient availability to plants.
Factors to consider when evaluating the pH of soils/media/water:
- Plants are always the best indicators of problems in the soil. Remember to look at roots as well as the shoots.
- As a grower, one should always conduct a soil/media analyses to determine the chemical characteristics of their growing media.
- Different plant species have different pH requirements.
- Different soil/media types will have different pH requirements.
- When recommendations for a specific pH are given, it is not necessary to waste time and money to attain the exact pH that is recommended. Usually a pH in the range of ± 0.5 pH units is adequate.
- Field soils (mineral soils) differ from potting media in that field soils are usually lower in organic matter (0.5 – 3.0%) and higher in micronutrients than potting media, which generally have high concentrations of organic matter (30-100%) and low levels of micronutrients. These factors will influence recommended pH values.
- Other factors such as soil environment, climate, and fertilizer type will interact with pH effects on plant growth and development.
Soil/water pH affects nutrient availability in primarily two ways:
- By increasing or decreasing the solubility of certain minerals.
- By affecting root integrity and thus the ability of roots to take up nutrients.
1) Effects of pH on mineral solubility
Field Soils
For most field soils the ideal pH range with regard to nutrient availability is between pH 6.0 and 7.5 (Figure 1), except for the acid-loving plants such as azalea, rhododendrons, etc. As the pH goes up, the availability of most micronutrients (iron, manganese, copper, boron, zinc, molybdenum) decreases because these elements combine with other elements such as calcium to form compounds of very low solubility. Likewise, as the pH decreases, micronutrient availability increases. In addition, below pH 4.0, the availability of aluminum and manganese can increase to toxic levels, causing root death.
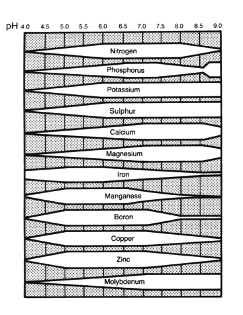
Figure 1. Nutrient availability in mineral soils as affected by pH. The wider the band for a particular element, the more available that nutrient is for plant uptake in the given pH range. (E. Truog. 1941-47.U.S. Dept. of Agr. Yearbook , p. 566-576).

Figure 2. Nutrient availability in organic planting media as affected by pH. The wider the band for a particular element, the more available that nutrient is for plant uptake in the given pH range. (K.A. Handreck and N.D. Black. 1999. Growing media for ornamental plants and turf. p. 86)
Potting Media
For most potting media, the ideal pH range with regard to nutrient availability is between pH 5.5 and 6.3 (Figure 2). However, as in field soils, crops such as azalea, rhododendron, etc. require a more acid medium. The primary problem with higher pH media is that iron is tied up in the organic complex at higher pH. At a lower pH, nutrients such as manganese, and sometimes iron and zinc are available at concentrations that are toxic to plants. Boron toxicity symptoms are also more likely to be expressed at lower pH, especially if irrigation water contains boron concentrations in excess of 0.50 ppm (mg/L).
2) Effects of pH on nutrient uptake
At low pH (<3.0) and high pH (>9.0) toxicity to roots can occur directly by extreme pH conditions due to the high concentration of hydrogen ions (H+) in acid conditions or hydroxyl ions (OH-) in alkaline conditions. Other factors will contribute to ‘pH toxicity’ even under less extreme conditions. As pH decreases (<5.0) in field soils, aluminum concentrations in soil solution increase, especially as pH drops below 5.0. This exposure of roots to aluminum is toxic, resulting in stunting and/or death of the root tips, which are the primary areas of the root where nutrient uptake occurs. In more organic media, such as those used for container production, elements such as manganese, boron, and sometimes iron can increase to high concentrations. This results in the excessive uptake of these nutrients to the point that they accumulate to toxic levels in the shoots and roots.
Don Merhaut is an Associate Extension Specialist for the ornamental, floriculture, landscape and nursery industries with UC Cooperative Extension at UC Riverside. He can be reached at donald.merhaut@ucr.edu.
