pH Management in Liquid Feed for Nursery and Greenhouse Production
“Maintain substrate pH around 5.5-6.4, and you will be fine.”
Dr. Paul Fisher, Environmental Horticulture, University of Florida
What is pH?
In practical terms, pH is a scale of how acidic or basic your irrigation or substrate water is. In scientific terms, pH is a measure of the activity of hydrogen ions (H+) in a water solution. This is approximated by the molar concentration of hydronium ions (H3O+) in solution because in water we don’t find free H+, but they are bound to a water molecule as hydroniums. A higher concentration of hydrogen ions creates a more acidic solution. This molar concentration in water is generally a very low value. For example, a concentration of 0.0000001 mol/liter of hydrogen ions is neutral, or equal to a pH of 7.
Moving down the pH scale indicates increasing concentrations of hydrogen ions, but each step represents a tenfold increase in concentration. For example, the hydrogen ion concentration required to decrease your pH from 6 to 5 is ten times larger than to decrease your pH from 7 to 6. This is the same as sweetening your coffee from very bitter to bitter with one spoonful of sugar. Then to sweeten it from bitter to semi-sweet it takes 10 spoonfuls, then 100 spoonfuls to make it from semi-sweet to sweet. I know, it’s weird. Since the molar concentration is always less than 1 and since each step of the scale counts ten times more concentration than the previous, pH is calculated as the negative logarithm of the molar concentration of hydrogen ions. This seems scary but it just means that if the concentration of H+ is 0.001 mol/liter (this is a very high concentration), the pH is 3; if the concentration is 0.00000001 mol/liter, the pH is 8. You can just count the zeroes!
How to measure pH?

There are various tools available to measure pH. The simplest one is a colorimetric strip. They are cheap, quick, and easy to use, and they provide a reasonable accuracy for liquid feed or substrate management. I like the fact that they don’t require calibration and never run out of batteries!
One step up in the sophistication scale is a hand-held pH meter. These are affordable ($100), portable, and resemble an EC meter. But they are more delicate and require more calibration than an EC meter. They only have a lifetime of a couple of years, but they have the advantage of giving you a number on a display. In comparison, the colorimetric strips require you to eyeball a color and make a guess on the pH measurement. Remember to order some calibration solution if you decide to buy a hand-held meter.
An even more sophisticated tool is an in-line pH meter. This meter can connect to a display that reads the pH of your irrigation water after fertilizer injection. These work great, but it’s always good to double check by taking a sample manually at the dripline. They also need regular maintenance and calibration.
There is a difference between measuring irrigation water pH and measuring substrate pH. The first is easy to measure, by collecting irrigation water into a container from the dripline, and then using your preferred pH measuring tool. The second is slightly more complicated. Recommended methods to measure substrate water pH include the pour-through method, the 1:2 method, the plug squeeze method, and the Saturated Media Extract method (see resources at the end of the article). Since pH is a logarithmic scale, the relatively small differences in dilution between the different sampling methods mentioned above have a negligible effect on pH measurements. Substrate pH is the recommended measurement for nutritional decisions, since it represents the pH experienced by plants.
How does pH affect plant nutrition?
In simple terms, a high pH (above 6.5) will usually cause microelements like iron and manganese to become less available, causing plant deficiency symptoms. This can be true even if you use iron chelates, since their availability is also pH-dependent and drops at high pH. This is by far the most common pH-related issue we encounter in greenhouse and nursery production (Figure 2). On the other hand, low pH can make some elements too available, causing iron and manganese toxicity.

The high pH deficiency versus low pH toxicity dilemma is modulated by the species of plants you are growing, due to the plant’s iron uptake efficiency. In blooming plants, species are customarily grouped as iron-inefficient (e.g. petunias) or iron-efficient (e.g. geraniums). Iron-inefficient plants prefer a low pH (5.4 – 6.0), and iron-efficient plants prefer a higher pH (6.0 – 6.6; Figure 3). Some practitioners refer to plants in the first group as the “Petunia group” and plants in the second group as the “Geranium group”.
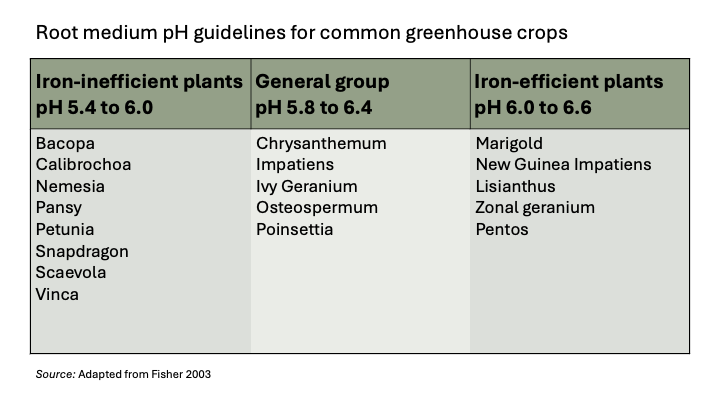
It is common to find high irrigation water pH (above 6.5) because water sources in California often have high alkalinity (high carbonate and bicarbonate concentrations). This causes a “liming effect”, particularly in periods of high evapotranspiration, when a lot of irrigation water is applied. Throughout the growing season, plants transpire water and leave behind carbonates and bicarbonates, that increase substrate pH. You can drop high pH by injecting phosphoric acid, nitric acid, N-pHuric, or sulfuric acid. It’s also common to use acidic fertilizers to counterbalance high alkalinity in the irrigation water.
Finally, the nitrogen form in your fertilizer affects substrate pH. Ammonium will decrease substrate pH, while nitrate will increase the pH, because plants exchange the first for a H+ and the second for a OH-. These end up in the substrate solution, and drop or raise pH. Over-irrigation with clear water also tends to leach salts away and raise the substrate pH.
Recommended pH ranges for many ornamentals are suggested in the Ball Red Book second volume: Crop Culture and Production and online for free on www.fertdirtandsquirt.com.
Azalea case study
Azaleas, rhododendrons, camelias, blueberry, heathers and other member of the ericaceous family are notorious for requiring low pH and for suffering from iron deficiency at high pH. In April 2024 we visited a grower in Riverside County that had scattered chlorotic plants in a block of azaleas in 1-gal containers. We quickly determined the reason for the chlorostic symptoms, by pouring about 25 mL of reverse osmosis water through 5 pre-irrigated pots and collecting the leachate solution at the bottom of each container. Chlorotic plants had high substrate pH, above 7.5 and healthy plants had pH between 5.5 and 6. These plants had been in the block for more than 3 months, so the liming effect of irrigation water may have played a role in rising pH. We recommended the grower to apply 21-7-7 fertilizer, a highly acidic fertilizer. Nevertheless, we were never able to pinpoint the origin of the random scattered pattern of chlorosis symptoms, perhaps when the substrate was prepared and lime was applied to peat, the substrate was poorly mixed before filling containers.


Figure 4. A block of azaleas showing pH-related micronutrient deficiency symptoms (left). A chlorotic plant (top right) next to a healthy one (bottom right).


Figure 5. Measuring pH in the pourthrough leachate of the healthy (left) and symptomatic plant (right).
The take-home message
Contrary to popular belief, a neutral pH of 7 is too high for plant nutrition! It will cause deficiencies, particularly in iron-inefficient species. The general suggested range is 5.6 to 6.4. Routine irrigation water and substrate water testing allows monitoring and correcting problems before symptoms show up.
Resources

- Nutritional and monitoring resources available at www.fertdirtandsquirt.com.

- Methods for substrate testing and link to video on 1:2 method
https://ag.umass.edu/greenhouse-floriculture/fact-sheets/methods-of-greenhouse-media-testing-how-they-differ

- Article on pourthrough method
https://content.ces.ncsu.edu/the-pour-through-extraction-procedure-a-nutrient-management-tool-for-nursery-crops
Gerry Spinelli is a Production Horticulture Advisor with UC Cooperative Extension in San Diego County. He can be reached at gspinelli@ucanr.edu.
Emma Volk is a Production Horticulture Advisor with UC Cooperative Extension in Ventura and Santa Barbara Counties. She can be reached at evolk@ucanr.edu.
Don Merhaut is an Associate Extension Specialist at UC Riverside. He can be reached at donald.merhaut@ucr.edu.
