Root of the Problem: Phytophthora in Ornamentals
Phytophthora species are recurrent pathogens infecting ornamental crops worldwide, with that, California is not the exception. From the disease diagnostics clinic from Del Castillo Lab, in 2024, Phytophthora root rot was the most prevalent disease diagnosed. Infected hosts include wax flower, gardenia, banksia, orchid rock rose, lavender, and boxwood, among other plants. From these plants, nine Phytophthora species were identified, with P. nicotianae, P. palmivora, and P. niederhauserii among the most common ones. For symptoms caused by these pathogens, visit the article from this newsletter.
Phytophthora: The Plant Destroyer

Phytophthora is a genus of destructive plant pathogens that affect many ornamentals. The genus belongs to the class Oomycetes, which are fungus-like organisms also called water molds. There are a dozen common species of Phytophthora that are routinely isolated from diseased plant material. Most of these species are polyphagous and infect many different hosts. There are two kinds of common symptoms: root rot and foliar symptoms. The first appear as brown or tan young roots, while the healthy ones are white. Root tissue can become rotten and soft, and a typical tell-tale symptom is that the surface layers of the root slough off like a sleeve, leaving behind the vascular tissue. The second type includes aerial symptoms like blight and dieback on leaves, stems, and flowers. In addition, foliar symptoms such as leaf necrosis, defoliation, and yellowing can also result from root rot, as the plants can’t uptake nutrients and water. Some species of Phytophthora can cause both symptoms. For example, P. nicotianae can give aerial blight in vinca (Catharanthus) and root rot in lavender (Lavandula). A good introduction to Phytophthora species that affect ornamentals can be found in Dr. Jenna Beckerman's article entitled “Phytophthora Diseases in Ornamentals” from Purdue University Extension, or scan the code below. For a review including a classification of Phytophthora species in eleven clades and the most common lifestyles, diseases caused, and host ranges of species in each clade, see: “Phytophthora: an ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation”, Brasier et al., 2022. There is also a great online resource, IDPhy (Abad et al., 2023), that contains resources for morphological and molecular identification of Phytophthora species.
Phytophthora Life Cycle
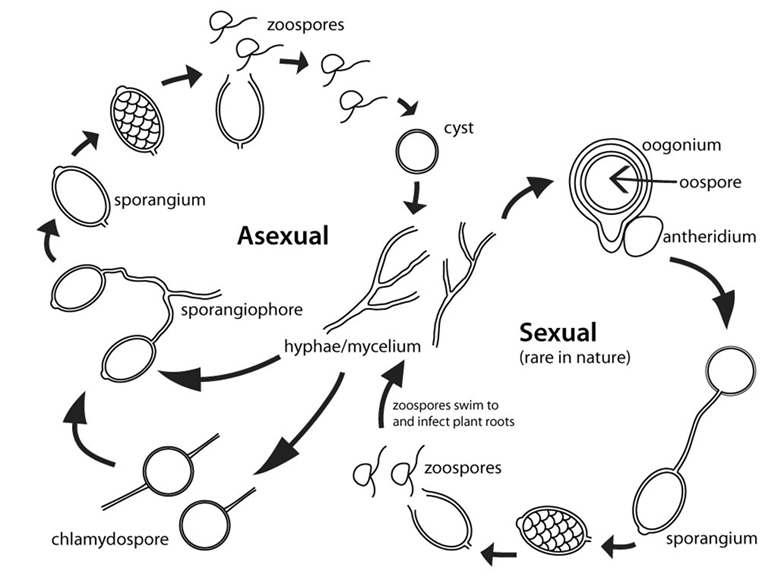
The phase hyphae/mycelium happen mostly inside the plant, after the cyst germinates and infects the plant’s cortical tissue. Sporangia then develop and release millions of zoospores. These are flagellate, i.e., they have two “tail-like” structures that allow them to swim (Figure 1). The primary dispersion mechanism in nurseries and greenhouses is through a continuous film of water between different container-grown plants. In nurseries and greenhouses, areas where water ponds or flows preferentially are common, and Phytophthora spread is often concentrated in these zones (Figure 2). Some species can develop chlamydospores; these are long-term survival structures that can have thick walls and survive in the soil for years. The pathogen develops chlamydospores in unfavorable environments and germinates when environmental factors become favorable. From a management standpoint, this represents a challenge, and extreme care should be taken to avoid soil contamination in the nursery (Figure 1).


Figure 2. Rosemary infected with Phytophthora sp. in an ebb and flow irrigation tub in a greenhouse (left) and Leucadendron in an outdoor nursery (right). Note that in both cases affected plants are associated with areas where water ponds.
How to manage Phytophthora pathogens
As a great number of Phytophthora species can infect a wide range of ornamental plants, selecting less susceptible or tolerant plant species is a challenge. Plant breeders have developed resistant varieties of popular ornamental species, but as many Phytophthora species can infect the same host, a resistant variety to one Phytophthora species can be susceptible to others. Given the reduced availability of plant material resistant to Phytophthora, the main tools to manage these pathogens are to implement strict and adequate sanitation practices during the production process of nursery stock.
Sanitation practices should be considered in all the steps and components of nursery production, starting with:
- Having pathogen-free sources of plant material. Incoming plant material entering the nursery may be infected with Phytophthora spp. and other pathogens. To eliminate any risk, it is recommended to propagate plant material following clean practices in your operation. If this is not feasible, source your plants from nurseries that follow best management practices. When new plant material is coming to the nursery, monitor all batches for symptomatic plants, place them in a quarantine area far away from your propagation stock, and do not mix new plant material with plants grown at your nursery. This step helps to prevent pathogen spread in case the plant material is infected.
- Clean and disinfect sources of substrate and pots, as these may harbor inoculum from previous crops. It is not recommended to reuse substrate, but in case it is done, it must be steamed as well as reused trays and pots. Some operations never reuse materials since the cost of steaming containers is close to the cost of new containers. Nevertheless, it is recommended to monitor new nursery containers, as it is not unusual to find plant material, soil, substrate, or other sources of contamination in new containers (Figure 3). Other facilities steam substrate every time it gets delivered to the nursery (Figure 3). This is an onerous practice, but it’s the only way to ensure that new inoculum is not introduced into the nursery.
- Maintain good sanitation practices by regularly cleaning and disinfecting floors, benches, greenhouse structures, and using boot mats for sanitation of footwear. In some greenhouses, workers are allowed to travel only in one direction, from the cleanest to the dirtiest areas.
- Accreditation to Improve Restoration (AIR). As a response to prevent the introduction of Phytophthora species in native California ecosystems, an accreditation program was created, with the initiative of the Phytophthoras in Native Habitats Work Group (PWG) –a group of restoration nurseries, land management agencies, researchers, and non-profit organizations. The AIR program is based on a systems approach that considers all the steps and components of plant production to exclude Phytophthora from native nurseries. To learn more about this program, read the article in this newsletter.
All these measures obviously increase labor costs and are only seldom applied by commercial nurseries. Nevertheless, strict and good sanitation practices are the best approach to reduce and prevent the presence of these pathogens in nursery production.


Figure 3. Steam equipment used by a nursery to steam incoming substrate (left) and an example of a pallet of brand-new nursery containers that were delivered with dead leaves and other plant material (right).
Importance of having good irrigation practices for Phytophthora disease management
Cultural management to prevent Phytophthora spp. in nurseries is mostly related to managing irrigation and runoff water, due to their prominent role in disseminating Phytophthora in the nursery and greenhouse. Ebb-and-flow and capillary mat irrigation systems, despite their advantages in terms of labor savings, particularly for small container sizes, are the worst irrigation method to employ. These methods provide a continuous film of water connecting a large number of containers on a bench or in a tub, with water moving between them. Indeed, they seem designed to provide the best environment for Phytophthora dissemination. Overhead sprinkler irrigation is the second-worst choice for dissemination due to the splashing effect of sprinkler drops and the runoff created by drops that do not land on pots. This effect can be alleviated if the containers are placed on gravel, since this generally avoids the development of standing water. Spray-stake or drip irrigation is the best choice, but care must be taken not to overirrigate, since leachate from one container can disseminate inoculum to the neighboring ones.
It is recommended to place containers on benches instead of on the ground and to use well-drained metal or plastic benches (instead of wood, which more easily harbors pathogens). Hanging basket production over benches in greenhouses can be problematic, as leachate from older, infected plants may drip onto younger plants below, continually re-inoculating the disease.
For outdoors nurseries or high tunnels where the use of benches is not justified, the choice of ground cover is very important in limiting the dissemination of inoculum. Gravel is preferred over weed mat. Plants should never be placed on bare soil. Some growers at nurseries that use weed mat as ground cover, place sensitive plants on metal wire stands (Figure 4). While suspending containers on stands certainly limits inoculation from leachate and runoff from the bottom of the container, it is unclear how tall the stands need to be to avoid the splashing effect from overhead irrigation.


Figure 4. Calibrachoas in a greenhouse and Osteospermums in an outdoor nursery suspended on a wire stand to limit Phytophthora spp. dissemination.
Chemical Control
A preventative chemical control program is the most common method used by commercial nurseries to fight Phytophthora spp. However, we recommend putting more emphasis on the sanitation, disinfection, and cultural practices described above. Phytophthora control compounds can be grouped into different generations based on their mode of action and time of introduction. Copper has been used for the control of Phytophthora since the early 1900s. The first generation of compounds has been used since the 1970s and includes phosphites and phosphonates. The second generation emerged in the late 1990s with the introduction of mefenoxam (e.g., Ridomil Gold, later Subdue Max). Third-generation compounds, introduced in the 2000s, include cyazofamid, oxathiapiprolin, and ametoctradin + dimethomorph (e.g., Segway, Segovis, Orvego). Although often referred to as “fungicides”, these compounds do not eradicate the pathogen’s propagules but only inhibit their ability to infect and to sporulate. In other words, these compounds are fungistatic, not fungicidal. This is a challenge when nurseries apply regular preventative chemical control programs, because often symptoms do not develop, and the presence of inoculum remains unnoticed. This limited awareness leads to little focus on sanitation and water management practices and ultimately to reintroduction and recirculation of pathogens in the nursery.
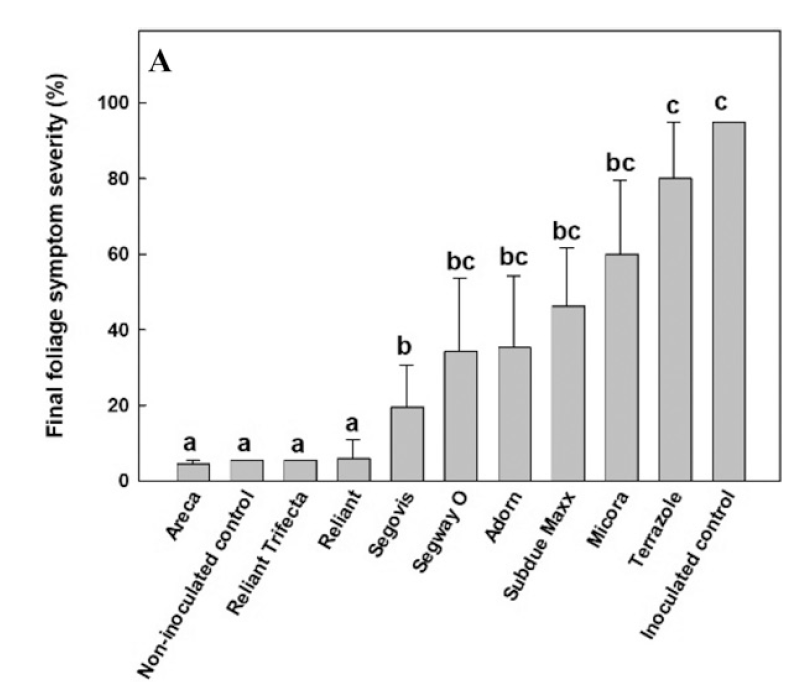
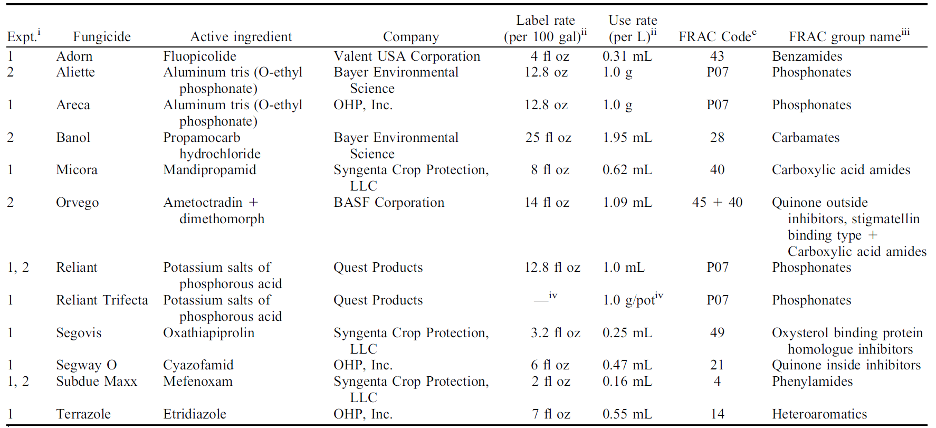
Resistance to fungicides has been reported for Phytophthora spp. in the case of mefenoxam. On the other hand, compounds like phosphonates (Areca, Reliant, and Trifecta) remain very effective in controlling the pathogen (Figure 5). Nevertheless, it is recommended to rotate regularly compounds with different FRAC codes to help prevent resistance development (Figure 6).
Biological Control
Biological fungicides come from beneficial fungi or bacteria that combat plant pathogens in a gentler, more environmentally friendly way. These natural defenders, such as Trichoderma, Bacillus, Coniothyrium minitans, Gliocladium species, and Streptomyces, can work by outcompeting disease-causing organisms, directly attacking them, or helping plants strengthen their own defenses (Paulitz & Bélanger, 2001).
Researchers and commercial companies have developed microbial inoculants that promote plant growth and boost resistance, showing real promise in managing Phytophthora root rot (Baysal-Gurel et al., 2019). In controlled studies, beneficial microbes have performed well, such as Burkholderia species suppressing Phytophthora nicotianae on Madagascar periwinkle (Catharanthus roseus), and Trichoderma harzianum reducing the pathogen by over 84% in Crossandra (Sonavane & Sriram, 2021).
In the ornamental plant industry, biological products are used to manage Phytophthora and similar pathogens. Typically applied as a soil drench before planting plug trays or cuttings, these products are preventative; they establish beneficial microbes in the root zone before disease takes hold. Trichoderma-based treatments, additionally, colonize infection-prone areas and reduce the chance of root rot.
While these biological options show great promise, it’s important to know that their performance can vary by climate and more trials are needed to assess how well they work under California’s specific growing conditions.
References
- Abad, Z. G., Burgess, T. I., Redford, A. J., Bienapfl, J. C., Srivastava, S., Mathew, R., & Jennings, K. (2023). IDphy: An international online resource for molecular and morphological identification of Phytophthora. Plant Disease, 107(4), 987-998.
- Baysal-Gurel, F. et al. (2019). Comparative performance of fungicides, biofungicides, and plant defense inducers in controlling Phytophthora root rot in flowering dogwood. Plant Disease, 103(7), 1703–1711.
- Brasier, C., Scanu, B., Cooke, D., & Jung, T. (2022). Phytophthora: an ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA fungus, 13(1), 12.
- Beckerman, J., and Creswell, T. (2020). Phytophthora Diseases in Ornamentals. Purdue Univeristy Extension. https://www.extension.purdue.edu/extmedia/BP/BP-215-W.pdf
- Dlugos, D. M., Bridges, W. C., & Jeffers, S. N. (2025). Managing Phytophthora Root and Crown Rot on English Lavender in the Greenhouse with Fungicides. HortScience, 60(3), 446-454.
- Paulitz, T. C., & Bélanger, R. R. (2001). Biological control in greenhouse systems. Annual Review of Phytopathology, 39, 103–133.
- Sonavane, P., & Sriram, S. (2021). Trichoderma harzianum (84.19%) and Streptomyces viridobrunneus (81.44%) were most effective in reducing Phytophthora nicotianae growth on Crossandra. Journal of Eco-friendly Agriculture, 16(2), 197–200.
