What We See in the Lab:
Disease diagnostics in ornamentals during 2024
The disease diagnostics service for greenhouse and nursery crops was initiated in California in 2020 by Del Castillo Lab at UC Davis. This service was free of charge thanks to funding from the Plant California Alliance (PCA) in the first two years and then by the National Plant Diagnostics Network (NPDN). Since the diagnostics service was launched, the number of samples submitted per year has increased more than threefold. In 2024, we processed 120 ornamental and vegetable transplant samples from 20 counties across California, ranging from Tehama County in the north to San Diego County in the south (Figure 1).
The diagnostics service provides direct valuable information to growers that submit samples, while also enabling us to monitor the prevalence and distribution of recurrent and emerging pathogens in the state. The information we gather from the diagnostics clinic helps inform the broader nursery industry about the most recurrent pathogen challenges in California. In this article, we focus on the diagnostic results from ornamental samples, which accounted for 86% of all submissions to the disease clinic.
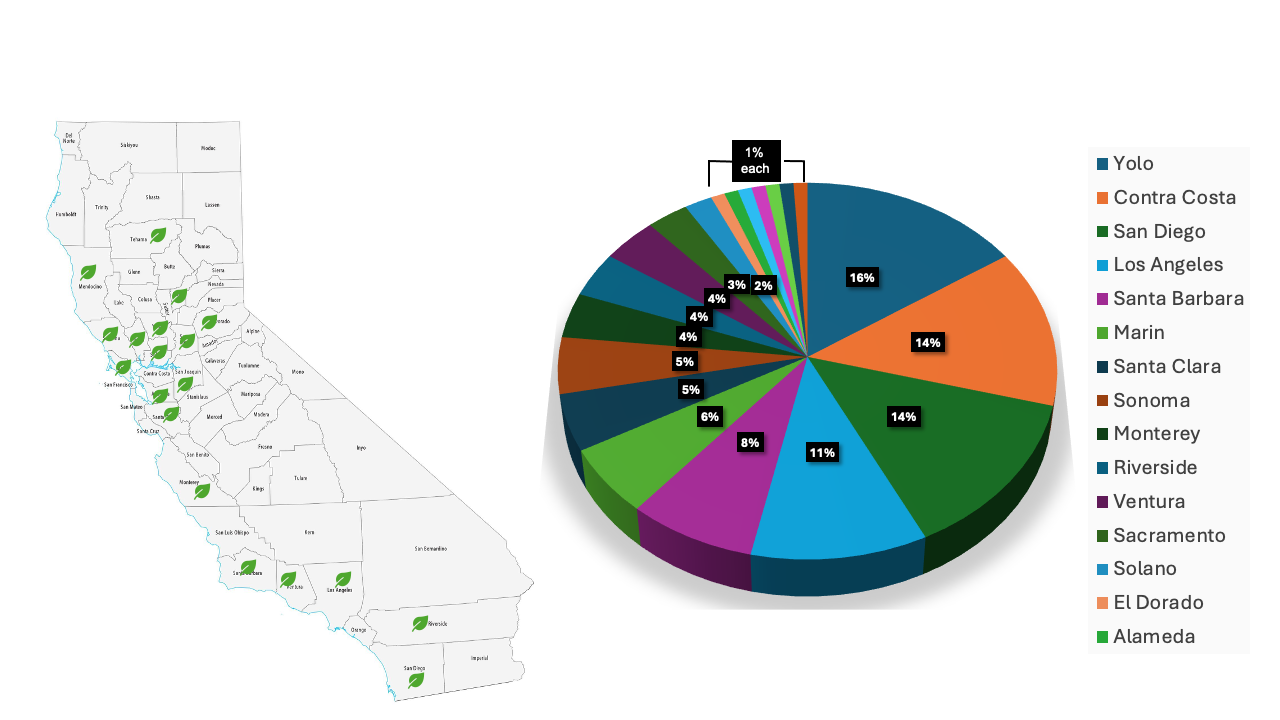
Most of the samples were submitted from Contra Costa, San Diego, and Los Angeles Counties, followed by Santa Barbara, Marin, and Santa Clara. Unique samples were also submitted from Napa, San Joaquin, Tehama, and Yuba Counties (Figure 1).
Samples received represented 62 different plant genera, with the majority (74%) of them being from a unique plant genus, including Petunia, Penstemon, and Anigozanthos, to name a few. Some plant genera from which multiple (2 to 6) samples were submitted include Arctostaphylos, Heteromeles, Quercus, Frangula, Lavandula, Asclepias, Phaleonopsis, Salvia, Gardenia, Pelargonium, and Rosmarinus. The heterogeneity of plant genera submitted showcases the wide diversity of the ornamental industry in the state.
Common disorders observed
By disorder, the most common symptoms were root rot (38%), leaf lesions (35%), and dieback (17%) (Figure 2). Samples with multiple symptoms: root rot and leaf lesions, stem and collar/crown rot and root rot and stem lesions were also received at a lower extent (1 to 3%). From now on, we will describe the diseases detected based on plant disorders.
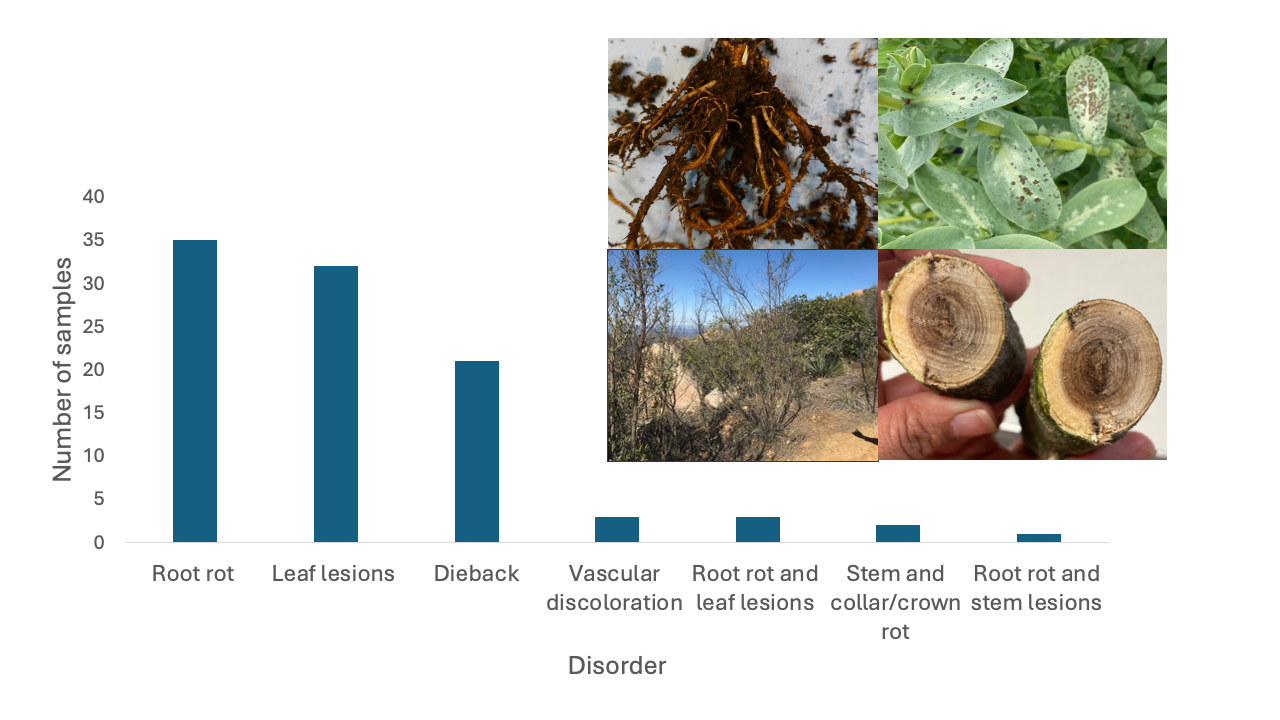
Diseases by disorder
Root Rot
- Phytophthora root rot was the most (57%) prevalent disease diagnosed from samples submitted from Los Angeles, Sacramento, San Diego, Sonoma, Ventura, and Yolo Counties. This disease was diagnosed from wax flower, gardenia, banksia, orchid rock rose, lavender (various cultivars), boxwood, among other plants. Symptoms observed include yellowing, leaf necrosis, defoliation, stunting, dieback, reduction of root biomass, and root and crown rot (Figure 3). From these hosts, we identified nine different Phytophthora species, including P. nicotianae, P. palmivora, and P. niederhauserii. Less commonly recovered species included P. cactorum, P. cinnamomi, P. cryptogea, P. persiana, P. tentaculata, and P. tropicalis (Figure 4).

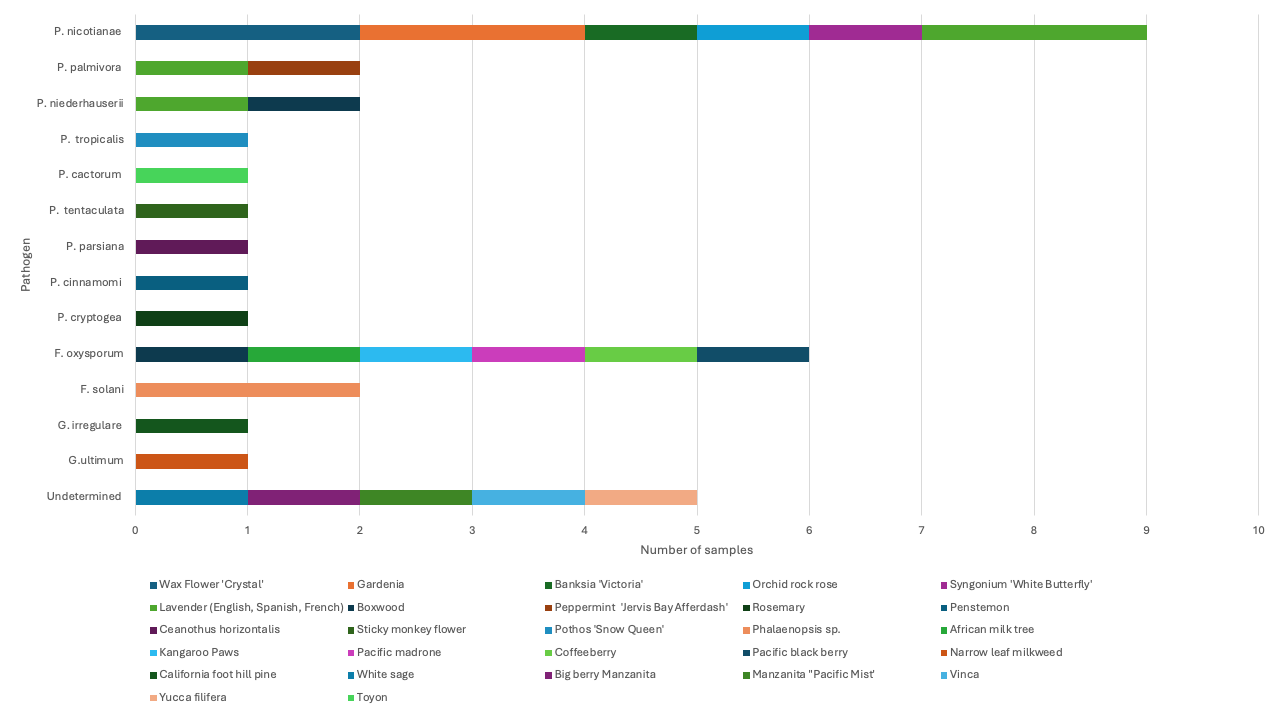
- Fusarium root rot was the second most prevalent disease, accounting for 15% of incoming samples from Contra Costa, Monterey, Santa Barbara, and San Diego Counties. Various Fusarium pathogens can cause root rot, the ones we identified include Fusarium solani species complex infecting orchids (Phaleonopsis) (Figure 5), and Fusarium oxysporum from Kangaroo Paws (Anigozanthos) (Figure 5) and African milk tree (Euphorbia trigona ‘Rubra’). Fusarium oxysporum was also recovered from symptomatic roots of California native plants such as Coffeeberry (Frangula californica), California blackberry (Rubus ursinus), and Pacific madrone (Arbutus menziesii). Nevertheless, F. oxysporum has not been reported as a pathogen of these species; to further confirm that the strains recovered cause root rot in these California natives, further pathogenicity assays should be conducted.

- Pythium root rot accounted for 6% of the samples with root rot disorder. The species Globisporangium irregulare and G. ultimum (formerly Pythium) were recovered from symptomatic roots of California foot hill pine (Pinus sabiniana) and from Narrowleaf milkweed (Asclepias fascicularis) samples submitted from Los Angeles and Monterey Counties respectively.
Leaf lesions
Leaf lesions were primarily caused by fungi (56%), followed by bacteria (19%), abiotic factors (12%) and insect damage (6%).
- Botrytis blight was the most common (19%) foliar disease, with samples having symptoms including brown lesions on the margin of the leaves, water-soaked lesions and leaf or needle blight (Figure 6).
- Other commonly diagnosed foliar diseases include Alternaria and Pseudomonas leaf spots, and abiotic factors, each accounting for 14% of cases.
- Less frequent diagnoses include Entomosporium, Boeremia, Colletotrichum, and Xanthomonas leaf spots. Symptoms of these diseases include enlarged water-soaked lesions, small circular brown or reddish spots on the top surface of the symptomatic leaves (Figure 7).


Dieback
Dieback caused by pathogens infecting woody and vascular plant tissues was diagnosed in tree samples submitted from Contra Costa, Napa, and Marin Counties. Some examples include:
- Pitch canker caused by F. circinatum was diagnosed from a Christmas tree sample (Pinus radiata) exhibiting discolored needles, wilted girdled branches, dieback, and brown discoloration of the stem beneath the bark surface (Figure 8).
- Botryosphaeria dieback caused by Botryosphaeria dothidea and Neofusicoccum sp. was diagnosed in Coastal redwoods (Sequoia sempervirens) with dieback symptoms and dark discolored wedge-shaped stains from a cross section of a tree branch (Figure 8).

Mixed infections
Several samples had multiple disorders, including a mix of root and foliar symptoms. Examples include:
- African milk tree (Euphorbia trigona ‘Rubra’) with Fusarium root rot caused by F. oxysporum, and sunken stem lesions from which Alternaria sp. was recovered (Figure 9).
- California foothill pine (Pinus sabiniana) seedling diagnosed with Pythium root rot caused by Globisporangium irregulare and Botrytis blight, showing blighted needles on the foliage (Figure 9).

What helps ensure a conclusive diagnostic?
A good sample submission is a key component for a successful disease diagnosis. When submitting samples:
- Provide as much sample information as possible (completing the plant disease form).
- Avoid sending completely dead plants, pathogens are often no longer present in completely necrotic tissue, as they move to healthy plant material to uptake nutrients. Isolations from dead plant material usually result in saprophytic microbes that are not the cause of the disease.
- Send entire plants when possible. In many cases foliar symptoms are a consequence of root, collar or stem pathogens, that won’t be recover from leaves.
- Send multiple plants showing a range of symptoms from less to more severe disorders.
- You can work together with the farm advisor in your area to select plant material to submit for diagnostics.
Acknowledgements
The disease diagnostics service is conducted by lead diagnosticians trained in the Del Castillo Lab. We thank Sophia Acker, Megan Gastelum, Ruchika Kashyap, Kayleigh Lampe, Sergio Gabriel Peralta, and Dean Watson for their dedication to sample processing and hypothesis-driven diagnostics.
