Soil and Water pH - Part 3
How do fertilizers affect pH?
The pH of soil and water and how it affects plant growth and chemical makeup of soils and media is somewhat detailed. In the next five newsletters, we will present different aspects about pH. Once complete, one should have a thorough understanding of pH and how it influences plant production.
Part I – What is pH?
Part II – How is nutrient availability affected by pH
Part III – How do fertilizers affect pH?
Part IV – How to adjust pH of soils
Part V – How to adjust pH of irrigation waters
Fertilizers may have a major impact on the pH of the media by either increasing or decreasing the soil solution pH. These effects are caused by two mechanisms: (1) Chemical effects - caused by the fertilizer coming in contact with the soil or media (2) Biological effects – caused by the reactions that occur when either the plant roots or soil microorganisms take up the nutrients.
Chemical Effects
Acid-Forming Fertilizers and Amendments
The following fertilizers will react with soil water or oxygen to form free hydrogen ions (H+). The term ‘free’ is a chemistry term that indicates that an ion, in this case H+, is not chemically attached to other compounds or soil material. The H+ ions are in the soil solution, resulting in a lower pH of the soil solution.
*Ammonium (NH4+)-based fertilizers (Equation 1). These fertilizers include products such as urea, anhydrous ammonia, and cottonseed meal. The ammonium (NH4+) reacts with oxygen (O) to form H+ ions.
Equation 1: NH4+ + 2O2 → 2H+ + NO3- + H2O
*Ferrous Sulfate (FeSO4). (Equation 2). The iron (Fe) reacts with water (H2O) to form H+ ions.
Equation 2: Fe2+ + 2H2O → Fe(OH)2 + 2H+
*Elemental Sulfur (S). The sulfur reacts with oxygen (O) and water (H2O) to form sulfuric acid (H2SO4). This compound dissociates (the elements chemically separate from each other) to form H+ ions (Equation 3).
Equation 3: 2 S + 3 O2 + 2 H2O → 2 H2SO4 → 2H+ + 2SO4-2
*Aluminum (Al)-based fertilizers (Equation 4). Aluminum (Al) reacts with water (H2O) to form H+ ions (Equation 4).
Equation 4: Al3+ + 2H2O → Al(OH)2+ + 2H+
*Organic matter. Organic matter that is low in base-forming compounds [calcium (Ca), magnesium (Mg), potassium (K) and sodium (Na)], will break down to form free H+. These organic media include acidic-type peat mosses, pine needles, some sawdusts and leaf molds. The biological and chemical breakdown of organic matter results in an increase in inorganic and organic acids, which can dissociate to form H+ (Equation 5).
Equation 5: Organic matter → carbonic acids (H2CO3), sulfuric acid (H2SO4), nitric acid (HNO3)
H2CO3 → 2H+ + CO3
H2SO4 → 2H+ + SO4
HNO3 → H+ + NO3
Base-Forming Fertilizers
*Oxides – such as calcium oxide (CaO) and magnesium oxide (MgO).
*Hydroxides – such as calcium hydroxide [(Ca(OH)2] and magnesium oxide [Mg(OH)2]
*Carbonates – such as calcium carbonate (CaCO3) and magnesium carbonate (MgCO3).
All of these compounds react with the soil particles, exchanging the hydrogen ions (H+) off of the soil colloids and replacing them with either calcium or magnesium. The free H+ then reacts with the OH- to form water (Equations 7-8).
Equation 6: 2H+[soil particle] + Ca(OH)2 → Ca2+[soil particle] + 2 H2O
Equation 7: 2H+[soil particle] + Ca(HCO3)2 → Ca2+[soil particle] + 2 H2O + 2 CO2
Equation 8: 2H+[soil particle] + CaCO3 → Ca2+[soil particle] + H2O + CO2
For a guide to fertilizers and their pH altering effects, please refer to the Western
Fertilizer Handbook (California Plant Health Association, 2002) (Interstate Publishers, 1-800-843-4774, Fax: 217-446-9706, email: info-ipp@ippinc.com)
Biological Effects
Biological effects on rhizosphere pH occur as a result of ions excreted from plant roots in response to the type of nutrients taken up into the roots. Other important reactions involved the biological conversions of nutrients from one form to another through microbial activity.
Acid-forming Reactions
*Excess cation uptake - If an excess of cations (positive ions) are taken up into the plant, relative to the uptake of anions (negative ions), positive ions may be released in the form of hydrogen ions (H+), which results in decreased rhizosphere pH. Nutrients that are taken up in the form of cations include calcium (Ca+2), ammonium (NH4+), potassium (K+), magnesium (Mg2+), iron (Fe2+), manganese (Mn2+), copper (Cu2+), zinc (Zn2+). Nutrients taken up in the form of anions include sulfates (SO4-2), phosphates (H2PO4-), nitrate (NO3-), molybdate (MoO4-).
*Nitrification of ammonium (NH4+) – Through microbial action (specific soil bacteria), ammonium (NH4+) can be converted to nitrate (NO3-). This process produces H+ ions (Equation 9).
Equation 9: 2 NH4+ + 4O2 → 2NO3- + 4H+ + 2H2O.
*Oxidation of sulfur (S) – Through microbial action, sulfur (S) in the form of H2S, reacts with oxygen to form sulfuric acid (H2SO4), which dissociates to form H+.
Equation 10: 2H2S + 4O2 → 2H2SO4 → 2H+ + SO4-2
Base-forming Reactions
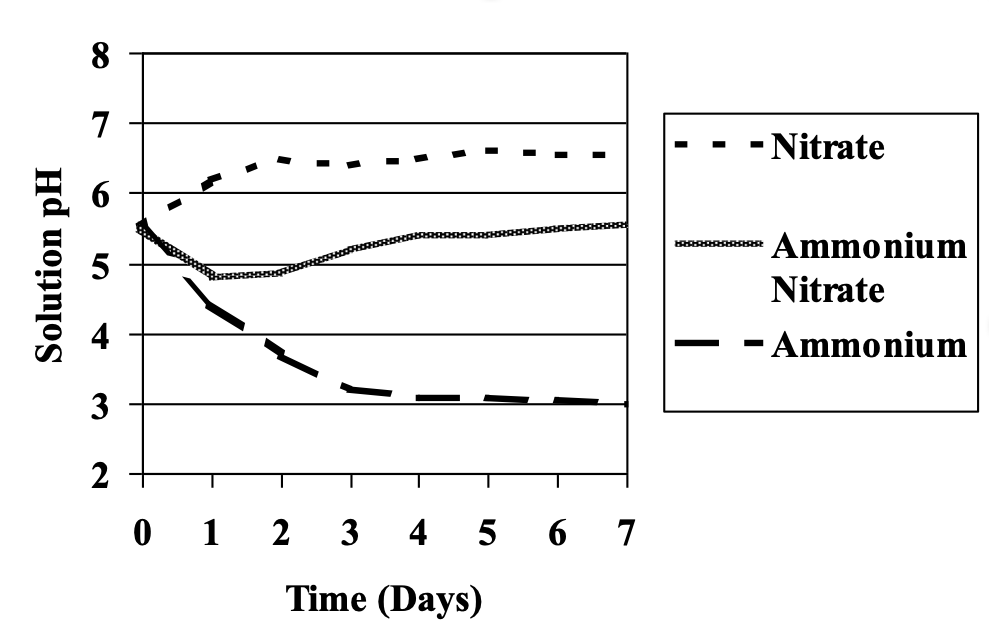
If anion uptake is higher relative to cation uptake, the result may be the release hydroxyl ions (OH-) from plant roots, resulting in an increase in rhizosphere pH. Since nitrogen accounts for approximately 70% of the total ions taken up, the form of nitrogen can have the most influence on this process. When nitrate (NO3-) is the predominant N form, OH- will be released resulting in an increase in pH (Figure 1). The rhizosphere pH will decrease when ammonium NH4+ is the predominant N form (Figure 1). When N is present as both NO3- and NH4+, the solution pH is relatively stable.